- Figure 1
- Figure 2
- Figure 3
- Figure 4
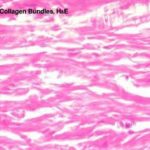
They are called white because they have a white color when fresh, called collagenous because on boiling they become hydrated and yield gelatin (glue). (Kolla=glue).
They are destroyed with weak acids and alkalis, and digested by pepsin and collagenase (which is an enzyme produced by the testis and some pathogenic bacteria).
With LM, they are arranged into wavy bundles. The bundles may branch, but the individual fibers do not. They are acidophilic, they stain pink with H&E; red with Van Gieson’s; green with Masson’s trichrome stain and blue with Mallory stain.
With EM, they are formed of bundles of microfibrils know as collagen fibrils. The fibrils are formed of tropocollagen molecules, and they have a characteristic periodicity repeated at 64 nm intervals.
This periodicity is due to the unique arrangement of tropocollagen molecules where they are arranged in end-to-end manner with each molecule overlapping the adjacent one by one quarter of its length.
Collagen is secreted into the intercellular matrix as tropocollagen molecules that polymerize to form collagen of 5 different types:
Collagen type I
It constitutes about 90% of total collagen in the body. It is found in fibrous connective tissue, skin tendon, ligaments and bone. The tropocollagen molecules are arranged to form fibers. Parallel collagen fibers are further arranged into strong bundles. These bundles are visible with LM and are responsible for the great tensile strength of this type.
Collagen type II
It is found in hyaline cartilage and consists of collagen fibrils dispersed in the ground substance.
Collagen type III (reticular fibers)
They form delicate supporting network in liver and lymphoid organs.
Collagen type IV and V
They do not form fibrils; type IV collagen is present in basement membranes and type V is found in small amount in most connective tissue.
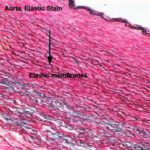
Elastin is a rubber-like material that is arranged as fibers and discontinuous sheets (in the wall of arteries). When present in sufficient number, elastic fibers give a yellow color to the fresh tissue (e.g., ligamentum nuchae of ruminants).
Elastic fibers are resistant to boiling and to hydrolysis by acid or alkali. They are also resistant to digestion by trypsin, but elastase from pancreas will digest it.
The elastic fibers can be stretched as much as 2.5 times their original length, to which they return when, released. They are found in organs whose normal function requires great elasticity such as vocal cords, lung, ligamentum nuchae, skin and arteries.
They are not identified in H&E sections but the large elastic fibers in elastic ligaments and the elastic sheets in arterial walls are seen as highly refractile light pink strands.
They can be selectively stains by Verhoeff’s stain, orcein (brown) and resorcin fuchsin (blue).
With EM, elastic fibers have two main components: Elastin that appears as an amorphous protein of low electron density. Microfibrils that are embedded in the periphery of the fibers and occurring in small fascicles in its interior.
They are synthesized by fibroblasts and smooth muscle cells as tropoelastin. The microfibrils are secreted prior elastin and provide scaffolding on which elastin forms fibers and sheets.
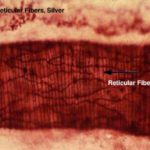
The reticular fiber form delicate network rather than coarse bundles around capillaries, muscle cells, nerve, adipose cells and liver cells. They also constitute the fibrous supporting tissue of endocrine, lymphoid and blood forming organs.
With LM, reticular fibers are not visible in H&E sections, but can be selectively stains black by silver impregnation (hence the term argyrophilic or argentaffin fibers) or with the periodic acid-schiff (PAS) reagent.
The argyrophilia of reticular fibers as well as their PAS reactivity reside mainly in the glycoprotein coat. When individual fibers are grouped to form collagen fibers, the coating is removed and argyrophilia disappear.
With EM, they are actually individual collagen fibrils (type III collagen) coated by glycoproteins. They have the same 64-periodicity typical of collagen fibrils.